COMPARATIVE ANALYSIS OF Y489 MUTANTS
This mutation site was located at the interior of the binding interface, and a hydrophobic binding groove was formed by surrounding amino acids T27, P456, C488, Q24 and F28. Therefore, the benzene ring from residue Y489 presented perfect geometric complementarity with this binding cavity. In addition, the polar Y83 and N487 at the top of the cavity formed hydrogen bonds with terminal chemical group -OH on Y489 (Figure 5A).
For amino acids ACDGHILMNPSTVE owning shorter sidechains, and the negative charged residues DE, all of them presented impossible preference to remedy the loss of binding modes in wild system (Figure 5B).
For both positive charged amino acids R489 and K489, incompatible properties were observed with the hydrophobic cavity, and this resulted in the protrusion out of sidechains from the cavity and destruction of the original bindin modes (Figure 5C). Although amino acids W489 and F489 were geometrically complemented, the heads still could not form hydrogen bonds with residues Y83 and N487 (Figure 5D).
Finally, no mutations with the potential to improve the binding affinities were not found in Y489 mutant systems.
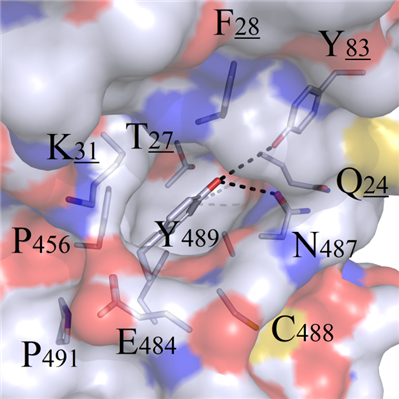 A
A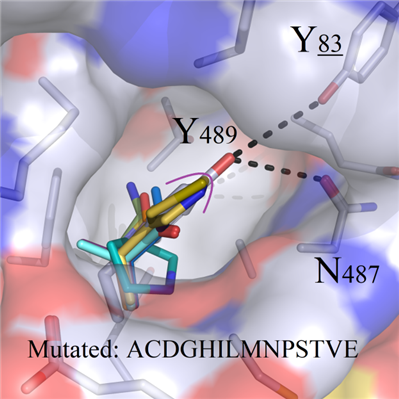 B
B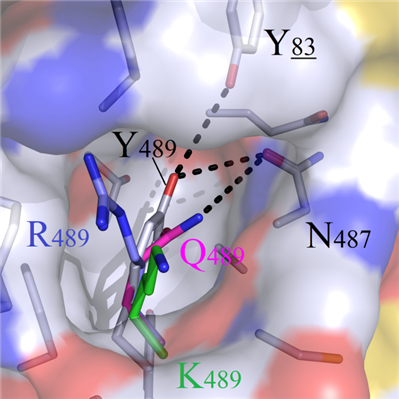 C
C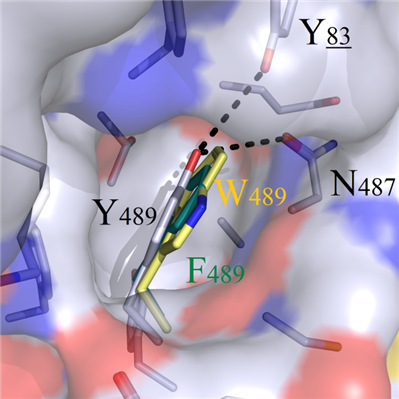 D
DFigure 5. Binding modes comparison of wild-type and Y489 mutant systems.