COMPARATIVE ANALYSIS OF F486 MUTANTS
For 19 mutated systems, the RMSD values fluctuated significantly from 0.635 to 0.789 as the mutation sites located at the core region of protein-protein binding interface. The physicochemical properties surrounding the mutated site F486 were analyzed as follows (Figure 3A):
(a) The upper part surrounded by amino acids L79, M82, Y83, P84, I88, I21 and A25 etc. formed one totally hydrophobic binding region.
(b) The bottom region contains polar amino acids N487 and Q24. However, one reasonable none-charged plane was formed between N487/Q24 themselves and Y83/Y489 residues, and no extra electric charges could be provided outwards to generate polarity with other residues.
Considering the binding characteristics of this regional pocket:
(a) Structure of amino acids with opposite charge characteristics (positively charged RHK, negatively charged DE, and polar non-charged STNQ) had experienced significantly swings as they could not match the original physical and chemical characteristics in wild-type complex(Figure 3B).
(b) In addition, due to the shorter sidechains of hydrophobic amino acids (CGPAVILM) compared with that of F486, the original geometric configuration in wild-type system could not be complemented, which led to the collapse of this binding region. All these elements resulted in the apparently structural changes and weakening of binding characteristics (Figure 3C).
(c) Hydrophobic amino acids (W486 and Y486) with longer sidechains not only could partially fill the cavity excavated by the wild amino acid F486, but also induced newly physical and chemical properties with surrounding residues(Figure 3D). Therefore, mutants F486W and F486Y were picked out for subsequent analysis.
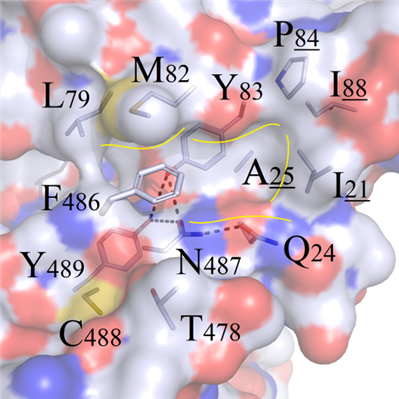 A
A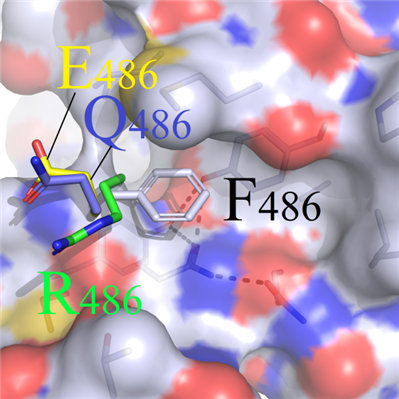 B
B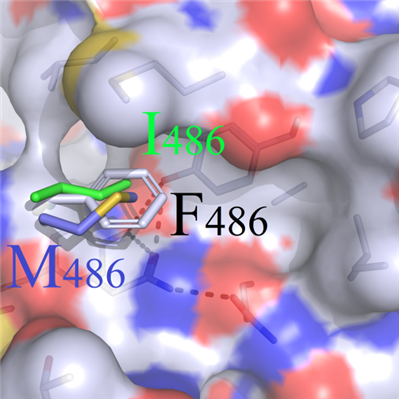 C
C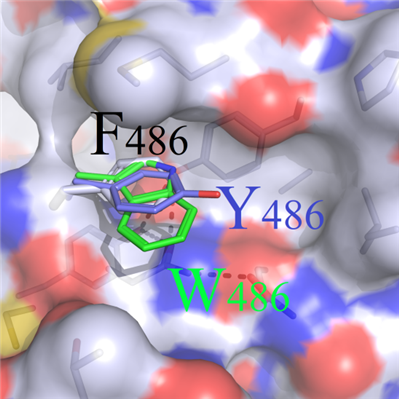 D
DFigure 3. Binding modes comparison of wild type and F486 series mutants. (A) Surface map of hydrophilic and hydrophobic properties around the F486 site (white: hydrophobic); (B) Selected residues: positive R486, negative E486 and uncharged polar Q486, compared with F486; (C) Hydrophobic short sidechain residues M486 and I486, compared with F486; (D) Hydrophobic long sidechain residues W486 and Y486, compared with F486;